 |
|
Supra-amphiphiles refer to amphiphiles that are constructed on the basis of noncovalent interactions such as electrostatic interaction, π−π stacking, charge-transfer interaction, hydrogen bonding, and host−guest interaction. Some dynamic and reversible covalent bonds such as imine and disulfide bonds, which are similar to noncovalent interactions under certain conditions, can also be used in the formation of supra-amphiphiles.
There are two main strategies for fabricating supra-amphiphiles according to the structure of the building blocks. One is to ‘create amphiphilicity' by the combination of the hydrophilic part and the hydrophobic part through noncovalent interactions or dynamic covalent bonds. And the other strategy is to ‘modulate amphiphilicity' by modifying conventional covalent amphiphiles with the techniques of noncovalent synthesis.
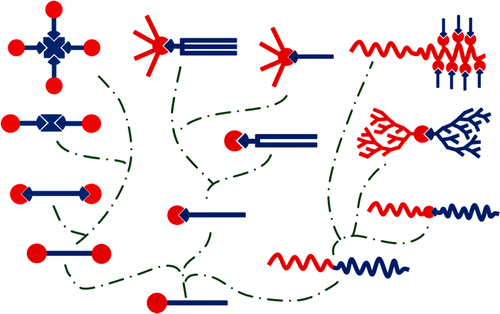
For amphiphilic species, topology dictates physical−chemical properties and applications. So far, various topologies of supra-amphiphiles have been achieved, such as single-chain head-to-tail, multi-chain head-to-tail and bola-form ones. Because of the convenience of noncovalent synthesis, the fabrication of supra-amphiphiles with some specific topology can be relatively less laborious and less difficult compared to that of their covalent counterparts.
Due to the dynamic nature of noncovalent interactions and dynamic covalent bonds, the supra-amphiphiles can be endowed with stimuli-responsiveness, changing the structures and properties under certain circumstances, when the specific moieties which are sensitive to pH, temperature, competitive components, light and enzymes etc. are introduced into the systems by rational design. Therefore, it is anticipated that supra-amphiphiles can be capable candidates in the areas of smart nanoscale carriers, chemical analysis, highly emissive smart materials, supramolecular photosensitizers with enhanced antibacterial efficiency and so on. |
|
 |
| Recent Publications: |
| Angew. Chem. Int. Ed. 2008, 47, 9049; Angew. Chem. Int. Ed. 2009, 48, 8962; Chem. Commun. 2009, 5380; Angew. Chem. Int. Ed. 2010, 49, 8612; Langmuir 2010, 26, 14414; Langmuir 2010, 26, 14509; Adv. Mater. 2010, 22, 2553; Langmuir 2011, 27, 14108; Langmuir 2011, 27, 12375; Small 2011, 7, 1379; Angew. Chem. Int. Ed. 2011, 50, 4952; Chem. Eur. J. 2011, 17, 3322; Chem. Soc. Rev. 2011, 40, 94; Langmuir 2012, 28, 14562; Langmuir 2012, 28, 14567; Polym. Chem. 2012, 3, 3056; Chem. Eur. J. 2012, 18, 8622; Langmuir 2012, 28, 10697; Langmuir 2012, 28, 6032; Acc. Chem. Res. 2012, 45, 608; Langmuir 2013, 29, 12375; Sci. Rep. 2013, 3, 2372; Angew. Chem. Int. Ed. 2013, 52, 8285; Chem. Commun. 2013, 49, 1808; Langmuir 2014, 30, 1531; Langmuir 2014, 30, 5989; Langmuir 2015, 31, 120; Chem. Sci. 2016, 7, 1151; ACS Appl. Mater. Interfaces 2016, 8, 4927; Adv. Funct. Mater. 2016, 26, 8920; Langmuir 2017, 33, 5879. |
|
| |
 |
|
|
|